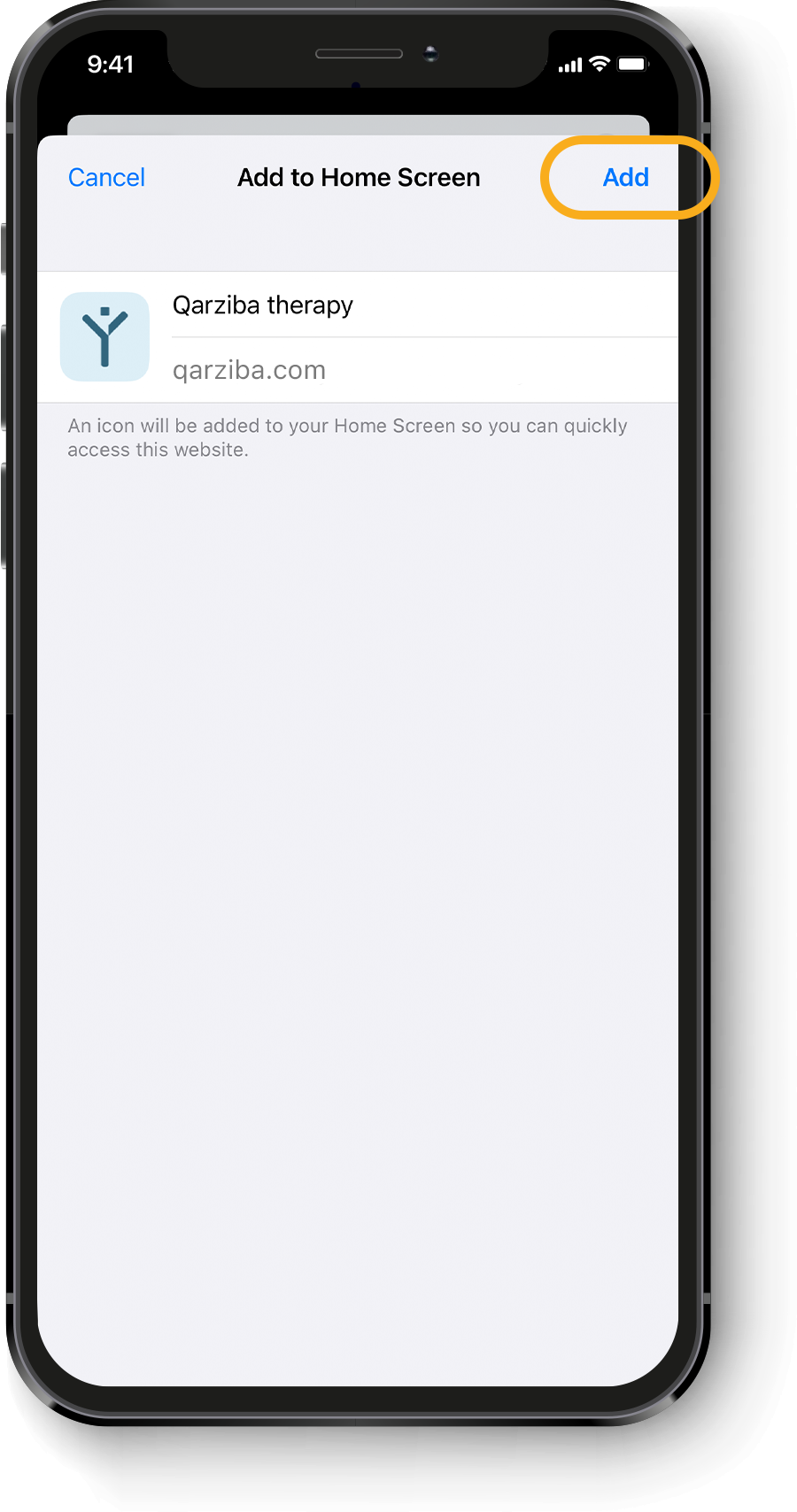
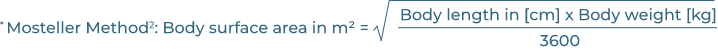Calculating the individual QARZIBA® dose1
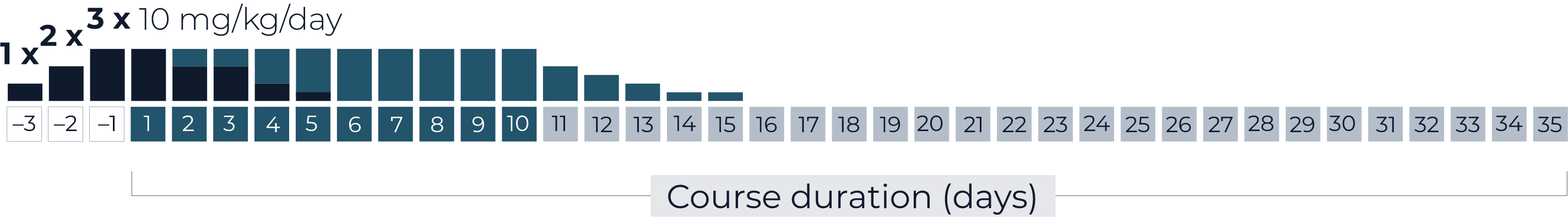
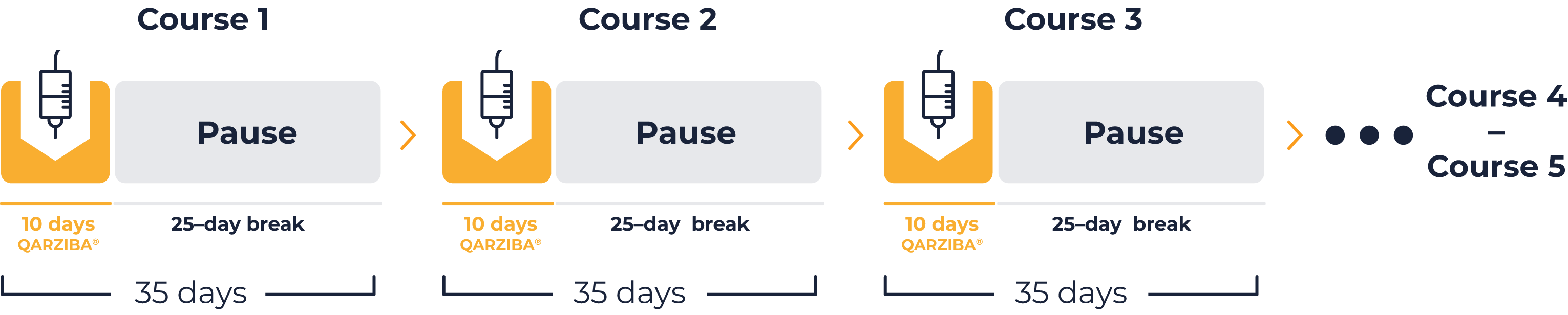
Cumulative dose per course
Cumulative dose 5 courses
(complete treatment)
Continue
1. QARZIBA® (dinutuximab beta). Summary of Product Characteristics. Available at:
https://www.ema.europa.eu/documents/product-information/qarziba-epar-product-information_en.pdf
Last accessed: March 2023. 2. Mosteller RD. N Engl J Med. 1987; 317: 1098.
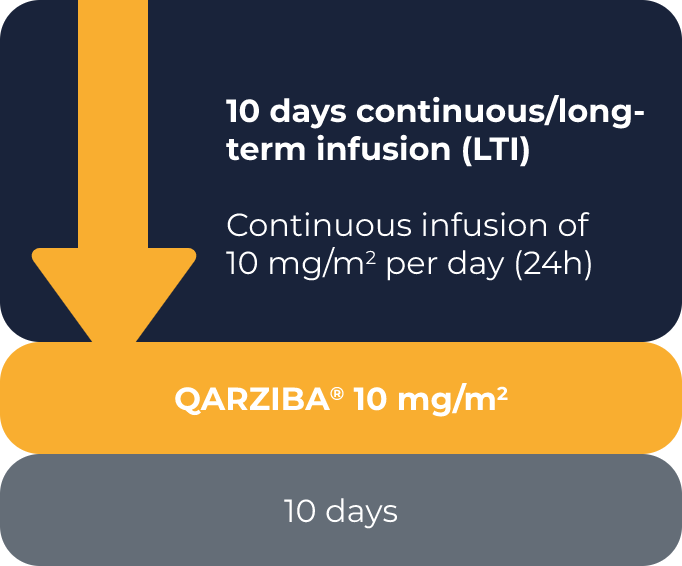
Selecting the infusion schedule1
Two infusion schedules are available:
• The continuous/long-term infusion (LTI) schedule

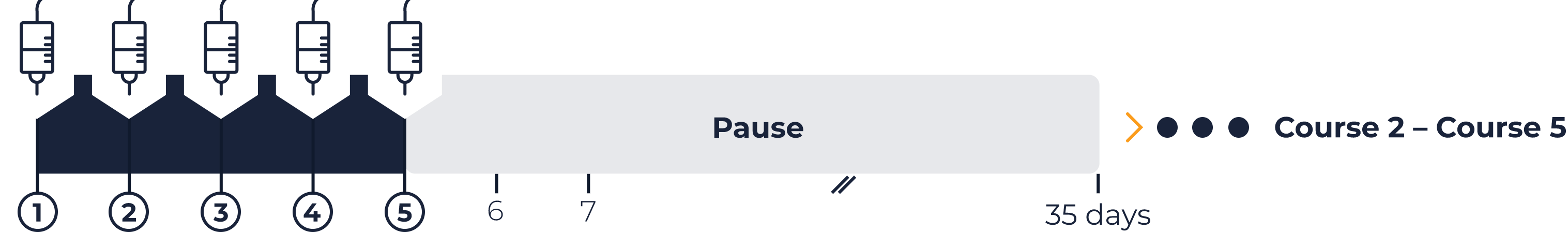
QARZIBA® infused continuously over the first 10 days of the 35-day course. Infusion rate = 2 mL per hour. Daily dose = 10 mg/m2.
• The short-term infusion (STI) schedule

QARZIBA® infused for 8-hours per day for the first 5 days of the 35-day course. Infusion rate = 13 mL per hour. Daily infusions = 20 mg/m2.
RECOMMENDED: The continuous/long-term infusion (LTI) schedule

Delivers the same total dose of QARZIBA® over a longer time period1

Reduces the incidence of clinically significant adverse events vs. the short-term infusion (STI) schedule2

Recommended by SIOPEN, the European neuroblastoma expert group3
Continue
Concomitant use of isotretinoin and IL-2 may be required. In patients with a history of relapsed/refractory disease and in patients who have not achieved a complete response after first line therapy, QARZIBA® should be combined with interleukin-2 (IL-2).1
10 days continuous/
long-term infusion
Continue
5 days
short-term infusion
Continue
1. QARZIBA® (dinutuximab beta). Summary of Product Characteristics. Available at:
https://www.ema.europa.eu/documents/product-information/qarziba-epar-product-information_en.pdf
Last accessed: March 2023.
1. QARZIBA® (dinutuximab beta). Summary of Product Characteristics. Available at:
https://www.ema.europa.eu/documents/product-information/qarziba-epar-product-information_en.pdf
Last accessed: March 2023. 2. Mueller I, et al. MABS. 2018; 10: 55–61. 3. Ladenstein R, et al. Cancers. 2020; 12: 309.
Long-term infusion schedule: Selecting the infusion device1
Short-term infusion schedule: Selecting the infusion device1
Two infusion devices are possible:
• Expert guidance recommends the first course is administered using 10x daily syringes, to assess tolerability and minimise the risk of product wastage.
• Once acceptable tolerability is confirmed, future courses can be administered using 2x 5-day infusion bags in a suitable pump.
Number of administrations to complete 35-day course:
10
5-day (120-hour) infusion bag
Number of administrations to complete 35-day course:
2
The short-term infusion schedule requires daily preparation of the solution for infusion.
The daily dose is 20 mg/m2, and the calculated dose should be diluted in 100 mL sodium chloride 9 mg/mL (0.9%) containing 1% human albumin.
You will need to select a suitable infusion device for delivery of these volumes at an approximate infusion rate of 13 mL per hour.
Please note, this is not the SIOPEN recommended infusion schedule and should only be used in exceptional circumstances due to the increased risk of AEs.2,3
Daily patient dose (10 mg/m2):
4,965 mg
(1,1 ml)
Daily patient dose (20 mg/m2):
4,965 mg
(1,1 ml)
Total course dose:
4,965 mg
(1,1 ml)
*Total volume infused should be 48 mL. 50 mL includes a 2 mL overfill estimate for dead volume.
The solution for infusion can be prepared in sufficient volume for 5 days of infusion, or on a daily basis. From a microbiological view, the solution for infusion should be used immediately, if not
used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would not normally be longer than
24 hours at 2 to 8ºC, unless dilution has taken place in controlled and validated aseptic conditions.1
From a microbiological view, the solution for infusion should be used immediately, if not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would not normally be longer than 24 hours at 2 to 8ºC, unless dilution has taken place in controlled and validated aseptic conditions.1
1. QARZIBA® (dinutuximab beta). Summary of Product Characteristics. Available at:
https://www.ema.europa.eu/documents/product-information/qarziba-epar-product-information_en.pdf
Last accessed: March 2023.
1. QARZIBA® (dinutuximab beta). Summary of Product Characteristics. Available at:
https://www.ema.europa.eu/documents/product-information/qarziba-epar-product-information_en.pdf
Last accessed: March 2023. 2. Mueller I, et al. MABS. 2018; 10: 55–61. 3. Ladenstein R, et al. Cancers. 2020; 12: 309.
Long-term infusion schedule: Preparing the solution for infusion1
Short-term infusion schedule: Preparing the solution for infusion1
Solution preparation depends on the volumes needed for the selected infusion device:
5-day (120-hour) infusion bag
Total
volume:
50 mL*
*Total volume infused should
be 48 mL. 50 mL includes a
2 mL overfill estimate for
dead volume.
Human
albumin (20%):
2.5 mL†
†The final solution for infusion
should contain 1% human
albumin.
QARZIBA® solution
(4.5 mg/mL):
1
This is your
patient specific
daily dose
Sodium chloride
(9 mg/mL (0.9%)):
10
Total
volume:
250 mL*
*Total volume infused should
be 240 mL. 250 mL includes a
10 mL overfill estimate for
dead volume.
Human
albumin (20%):
12.5 mL†
†The final solution for infusion
should contain 1% human
albumin.
QARZIBA® solution
(4.5 mg/mL):
1
This is your
patient specific
daily dose
Sodium chloride
(9 mg/mL (0.9%)):
10
Daily (8-hour) 100 mL device*
*Short-term infusion only. Not recommended.
Total
volume:
100 mL*
†Dead line volumes should
be accounted for based
on the device you are using.
Human
albumin (20%):
5.0 mL†
†The final solution for infusion
should contain 1% human
albumin.
QARZIBA® solution
(4.5 mg/mL):
1
This is your
patient specific
daily dose
Sodium chloride
(9 mg/mL (0.9%)):
10
The solution for infusion can be prepared in sufficient volume for 5 days of infusion, or on a daily basis. From a microbiological view, the solution for infusion should be used immediately, if not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would not normally be longer than 24 hours at 2 to 8ºC, unless dilution has taken place in controlled and validated aseptic conditions.1
1. QARZIBA® (dinutuximab beta). Summary of Product Characteristics. Available at:
https://www.ema.europa.eu/documents/product-information/qarziba-epar-product-information_en.pdf
Last accessed: March 2023.
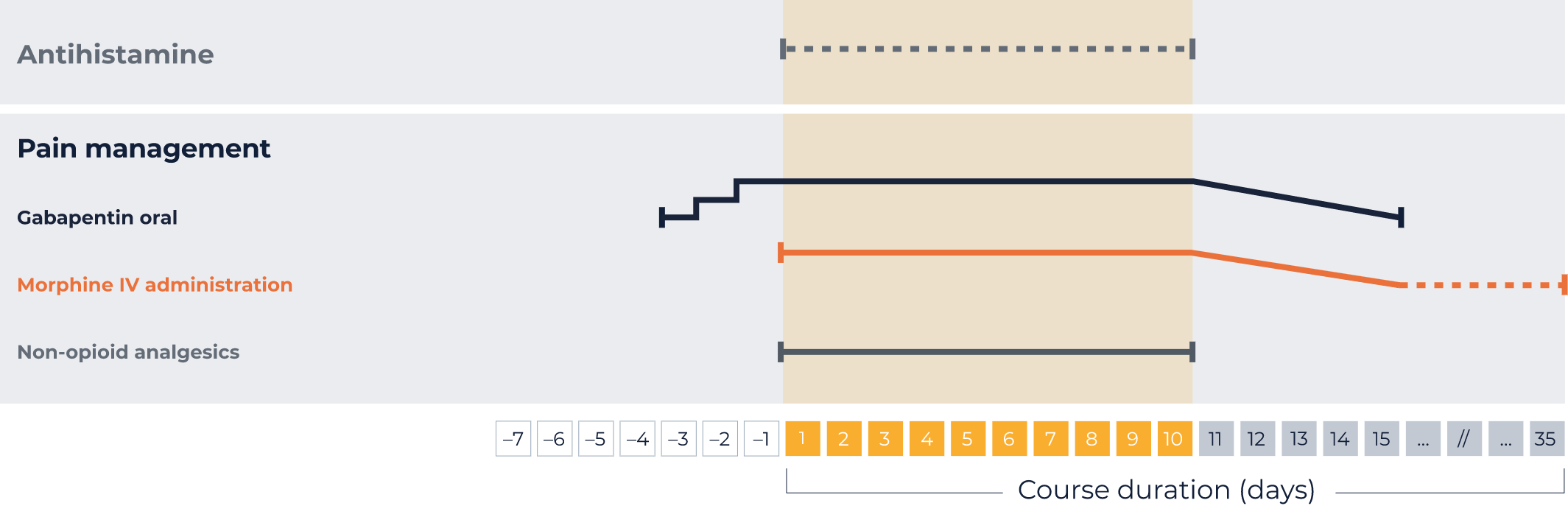
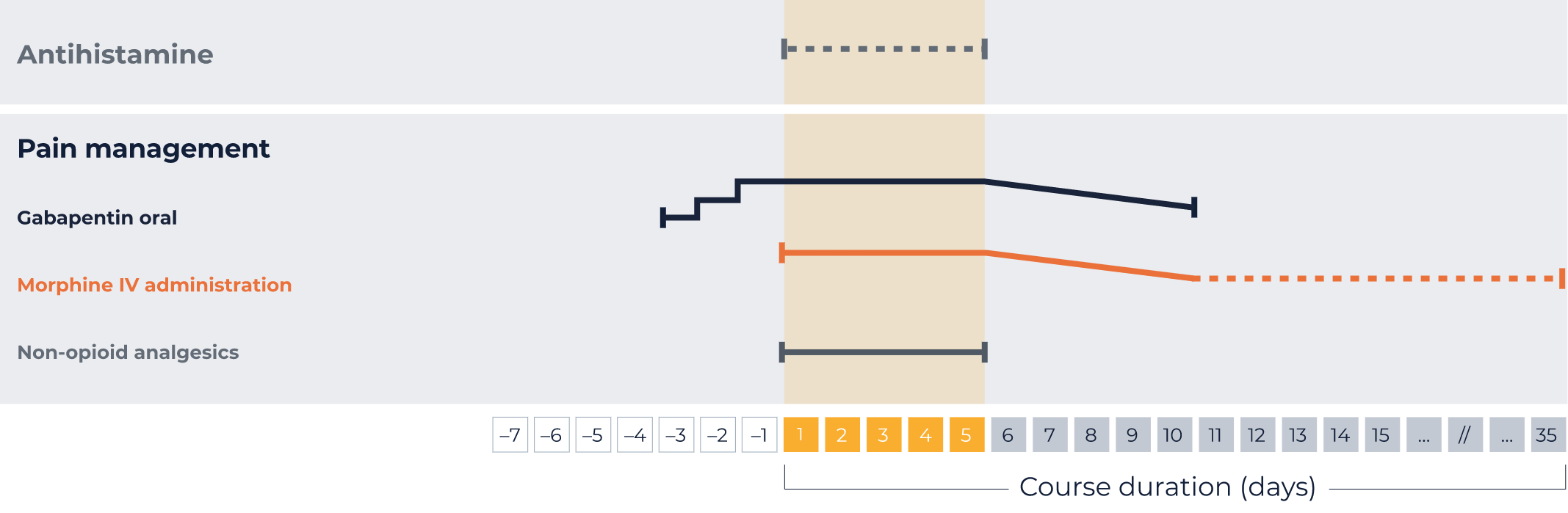
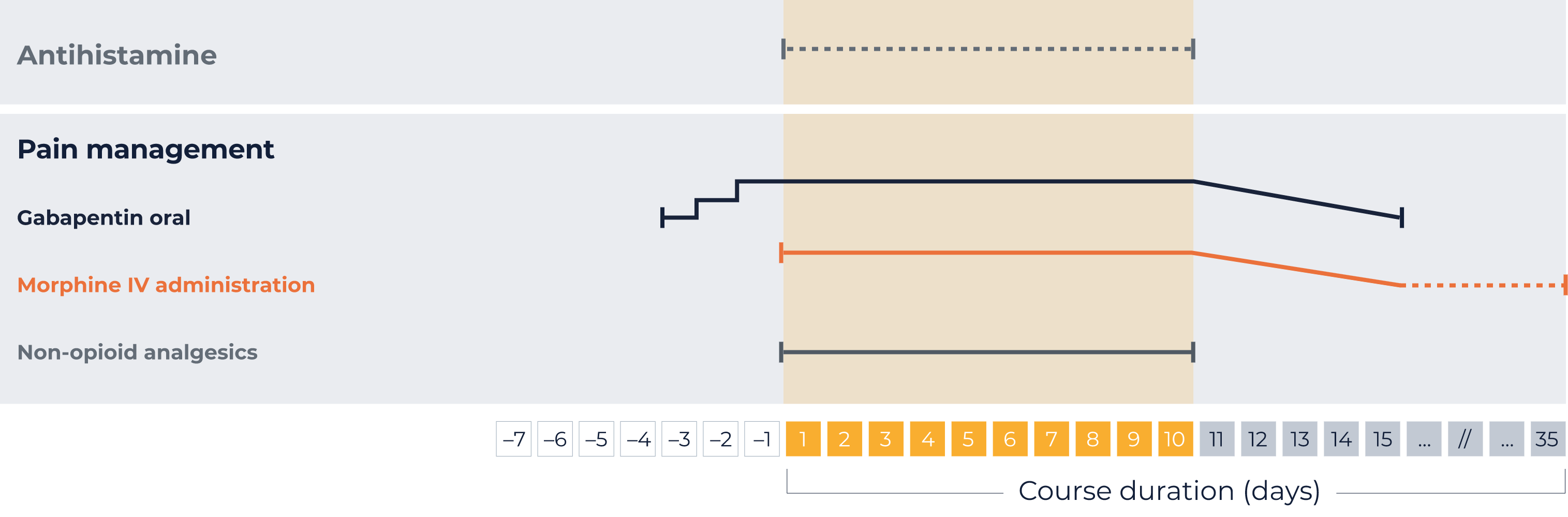
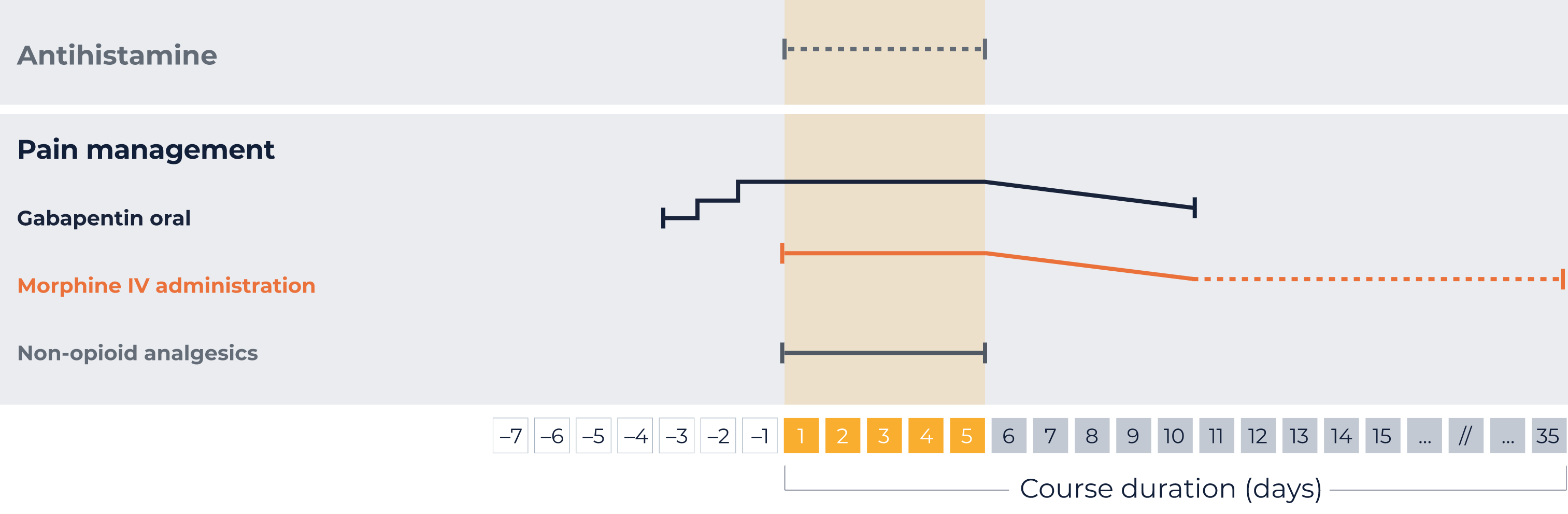
Your individual administration schedule: LTI1*
Pre- and concomitant medications:
QARZIBA® 4.9 mg per day
QARZIBA® 4.9 mg over 8h/day
Antihistamine
IV injection 20 minutes prior to each QARZIBA® infusion
Every 4–6 hours, as required, during QARZIBA® infusion
Gabapentin oral*
3 days prior to QARZIBA®: 1x 300 mg
2 days prior to QARZIBA®: 2x 300 mg
1 days prior to QARZIBA®: 3x 300 mg
Opioids*
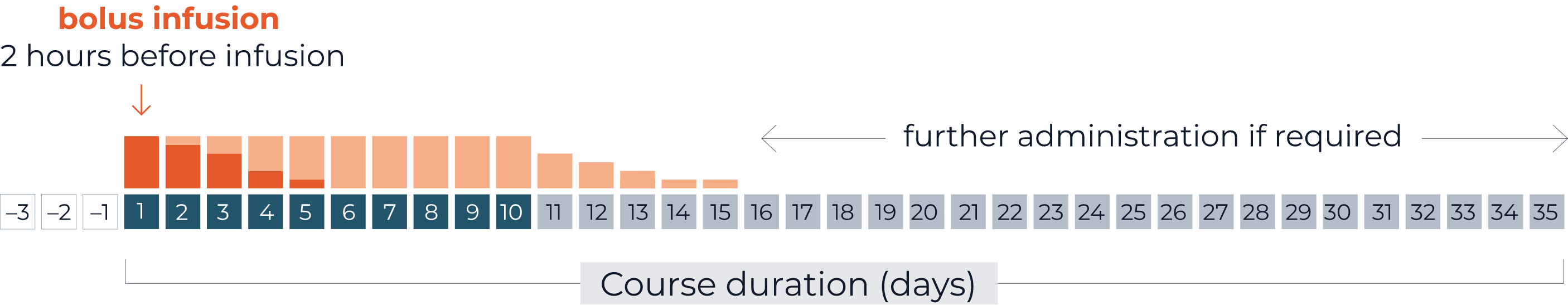
Morphin IV before
QARZIBA® infusion:
Bolus (0,204 - 0,510 mg/hour) 2 hours
before
During
QARZIBA® infusion:
Continue with 0,306 mg/h
With continuous infusion, in response to the patient’s pain perception, it may be possible to wean off morphine over 5 days by progressively decreasing its dosing rate (e.g., to 0.02 mg/kg/hour, 0.01 mg/kg/hour, 0.005 mg/kg/hour)
After weaning off intravenous morphine, in case of severe neuropathic pain, oral morphine sulphate (0.2 to 0.4 mg/kg every 4 to 6 hours) can be administered on demand. For moderate neuropathic pain, oral tramodol may be administered.
With daily infusions of QARZIBA®, morphine infusion should be continued at a decreased rate (e.g. 0.01 mg/kg/h) for 4 hours after the end of the infusion.
After weaning off intravenous morphine, in case of severe neuropathic pain, oral morphine sulphate (0.2 to 0.4 mg/kg every 4 to 6 hours) can be administered on demand. For moderate neuropathic pain, oral tramodol may be administered.
Non-opioid analgesics
e.g., paracetamol and ibuprofen
Permanently during QARZIBA® infusion
1. QARZIBA® (dinutuximab beta). Summary of Product Characteristics. Available at: https://www.ema.europa.eu/documents/product-information/qarziba-epar-product-information_en.pdf
Last accessed: March 2023. Administration of interleukin-2 if indicated.
IV, intravenous; LTI, long-term infusion.
*Your patient specific dose
1. QARZIBA® (dinutuximab beta). Summary of Product Characteristics. Available at: https://www.ema.europa.eu/documents/product-information/qarziba-epar-product-information_en.pdf
Last accessed: March 2023. Administration of interleukin-2 if indicated.
IV, intravenous; STI, short-term infusion.
*Your patient specific dose

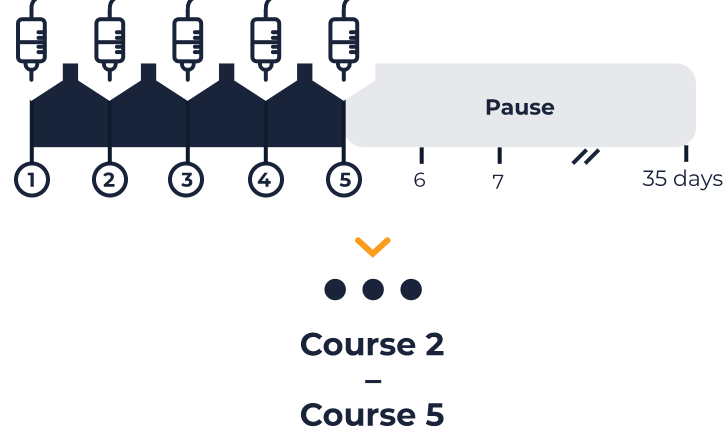
The following schedule is specific to each patient. It is based on the patient data you entered at the beginning.
The following schedule is specific to each patient. It is based on the patient data you entered at the beginning.
1x 102 mg/day,
2x 102 mg/day,
3x 102 mg/day
Maximum single dose is 300 mg. Dosing schedule should be maintained for as long as required.
Tapering off after weaning off IV morphine infusion, at the latest after QARZIBA® infusion has stopped.
Morphine IV administration
Morphine as bolus infusion of 0.02 to 0.05 mg/kg/hour morphine 2 hours before continuous QARZIBA® infusion.
Subsequently, a dosing rate of 0.03 mg/kg/hour is recommended concomitantly with QARZIBA® infusion.
If necessary, weaning off by the hour depending on the pain intensity.
On-demand opioid medication (after weaning off intravenous morphine)
- Severe neuropathic pain: oral morphine sulphate (0.2 to 0.4 mg/kg every 4–6 hours)
- Moderate neuropathic pain: oral tramadol
Non-opioid analgesics should be used permanently during QARZIBA® infusion, e.g., paracetamol or ibuprofen.